Entrepreneur Insight
Amsino, A Strong Force in the Fight Against COVID-19
By Melody Yuan
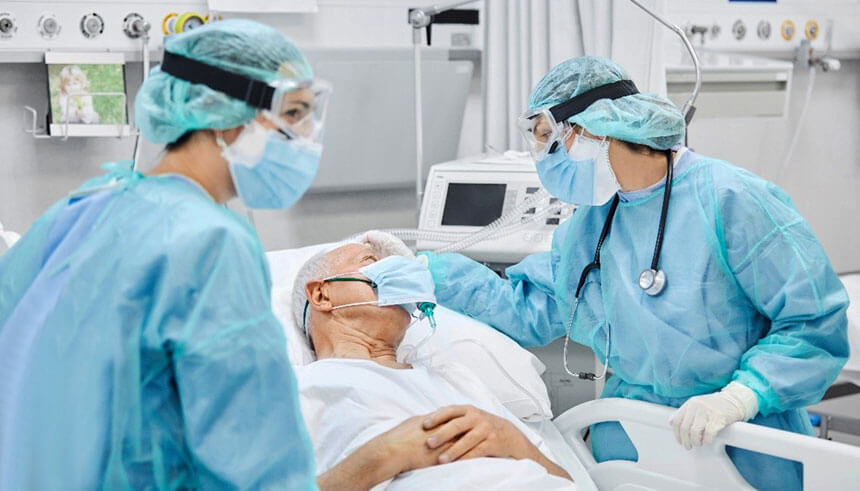
How a medical device company supported the frontlines through innovation during COVID-19
While COVID-19 came as a surprise for many and continues to wreak havoc across communities and businesses in the U.S., one medical device company was ready to assist in fighting off the pandemic. “COVID-19 has been a wake-up call across the world,” says Richard Lee, founder and CEO of Amsino International Inc., a medical device company founded in California in 1993. “I just saw a need for technological innovation and new methods for stopping the spread of infectious diseases, and our company mission is just that: improving the safety and efficacy of patient care.”
With many patented products and services from respiratory therapy systems to enteral feeding systems, Amsino creates and distributes most of their medical devices to hospitals and health care facilities. When the pandemic first hit in China, they kicked their production into high gear in anticipation of the disease hitting the U.S.
“Because COVID causes respiratory tract infections, we’ve seen a huge clinical need for multi-functional ventilators, filtered full-face masks, high flow oxygen delivery products, home medical equipment and closed mucus suction devices that help prevent the spread of the virus in hospital and nursing home settings,” says Lee. “Even in a post-COVID world, I think the demand for infection prevention products, especially in the respiratory care space, will grow substantially.”
Innovation never stops
According to the Centers for Disease Control, hospital acquired infections (HAI) in American hospitals alone account for 1.7 million infections and 99,000 associated deaths each year. To be more specific, one out of every 25 hospitalized patients in the U.S. is affected by HAI.
“That number is just too high,” says Lee. “And with the inevitable probability of a strong infectious disease after many warning signs with predecessors like SARS, H1N1 and Ebola, we knew that improving our medical devices, systems and processes was a race against time.”
“With the inevitable probability of a strong infectious disease after many warning signs with predecessors like SARS, H1N1 and Ebola, we knew that improving our medical devices, systems and processes was a race against time.”

When COVID-19 hit
“We knew that when the COVID outbreak hit China, that it would only be a matter of time before it would come for the U.S.,” recalls Lee.
To actively prepare for the fight against COVID, Amsino set up a COVID task force, sending urgently needed medical products to frontline hospitals in Wuhan, Shanghai and Jiangsu provinces. The company also donated more than 1 million RMB to support the medical industry in China tackling the crisis at hand. “After taking all necessary precautions, all of our Amsino plants in China resumed full production by the middle of February and worked 24/7 to then produce urgently needed medical products to ship around the world,” says Lee.
Once the COVID outbreak reached the U.S., Amsino joined forces with the Committee of 100, a non-partisan leadership organization of Chinese Americans, to donate personal protective equipment to the frontlines for essential workers, hospitals and health care facilities across America.
“Amsino has an extensive COVID product portfolio to help with the fight,” says Lee. From thermal imagining systems and infrared forehead thermometers, to bi-level positive airway pressure (BiPAP) ventilators, aerosol tubings, respirators, surgical masks and hand sanitizers, the list goes on with the number of medical products Amsino has contributed.
“The fight is far from over,” emphasizes Lee, “and we may see a decline in demand for immediate personal protection products once the pandemic dies down, but we think the demand for respiratory tract medical devices will remain strong.”

"The fight is far from over, and we may see a decline in demand for immediate personal protection products once the pandemic dies down, but we think the demand for respiratory tract medical devices will remain strong."
Overcoming growing pains
Today, Amsino has two R&D centers and four FDA-registered manufacturing plants in the U.S. and China. The company also has six business units serving the global market. What began as a medical device startup in 1993 quickly grew into an international company.
“East West Bank has been with us all the way during our growth,” says Lee. “We made a few acquisitions along the way, and the bank helped finance those acquisitions.” From acquiring Hospira Inc.’s global business for their RECEPTAL surgical suction product line in 2014, to Shanghai Regent Medical Technology Co. in 2019, Amsino has continued to grow at a steady pace. Managing multiple locations across borders, along with being on top of a constantly changing global medical regulatory landscape, came with its set of obstacles and growing pains.
“China has recently taken an overkill approach on certain regulations and enforcements,” says Lee. “It takes much longer now to get a medical device registered there than here in the U.S., largely due to clinical trial requirements.” This wasn’t the case a few years ago when China first opened up to the medical market. Today, a medical device company must register products according to class categories, provide supportive clinical data, and complete translations of packaging and labeling of the product. Registrations are valid only for five years, and the renewal process is just as time consuming.
"All Amsino facilities are FDA registered, and we operate in strict compliance with FDA regulations and the regulations in the countries we serve.”

Another area that gave Amsino growing pains was the management of a global supply chain. “Manufacturing offshore means a long lead time,” says Lee, “and to ensure our products always arrived on time, we established a demand planning system that takes a customer’s rolling forecast, production lead time and shipping time into account. This system has proven to work well, and we also keep a large inventory in our distribution centers in the U.S. and Asia so that we can meet customers’ lead time requirements.”
Amsino’s growth trajectory
“We’ve seen what we’re capable of during this pandemic, and I think we’ll continue to witness the accelerated growth of the company in the coming years,” says Lee proudly. “I see Amsino as a global market leader in infection prevention technologies, and we’re also aiming to be the leading company for digital surgical suction systems in the next five years.”
Amsino has already filed for 76 patents on their new generation of surgical suction equipment, 36 of which are invention patents. “Our first generation of surgical suction products has launched in the market,” says Lee, “and with the upcoming global launch of our new models, we’re uniquely positioned to lead the way in this area.”
Sign up for the Reach Further Newsletter
We’ll keep you in the know about the latest US-Asia business news and trends.
Suscríbase al boletín Reach Further
Lo mantendremos informado sobre las últimas noticias y tendencias comerciales entre Estados Unidos y China.